Artificial photosynthesis to provide solutions for carbon capture and conversion
Scientists have found a method to mimic nature’s process of reducing carbon dioxide in the atmosphere, namely photosynthesis, to capture excess carbon dioxide in the atmosphere.
Context
Scientists have found a method to mimic nature’s process of reducing carbon dioxide in the atmosphere, namely photosynthesis, to capture excess carbon dioxide in the atmosphere.
About the finding
- A team of Scientists from Jawaharlal Nehru Centre for Advanced Scientific Research, an autonomous institute of the Department of Science & Technology (DST), Government of India, led the discovery.
- They designed and fabricated an integrated catalytic system based on a metal-organic framework (MOF-808).
- It comprises a photosensitizer (molecules that absorb light and transfer the electron from the incident light into another nearby molecule).
- It can harness solar power and the catalytic center can eventually reduce CO2.
- Use:Artificial photosynthesis (AP) harnesses solar energy and converts the captured carbon dioxide to carbon monoxide (CO), which can be used as a fuel for internal combustion engines.
What are artificial photosynthesis and the process involved?
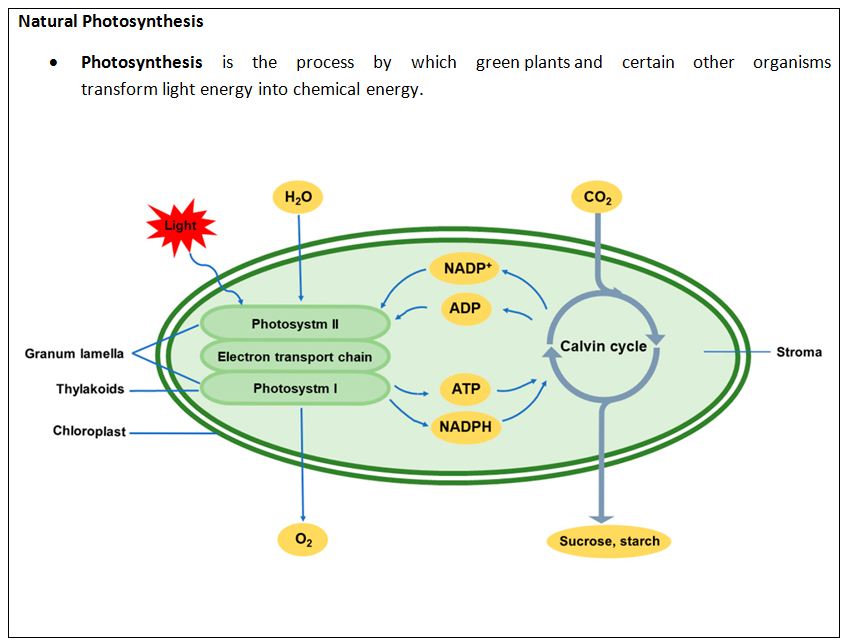
- Artificial photosynthesis is a chemical process that bio-mimics the natural process of photosynthesis to convert sunlight, water, and Carbon dioxide into carbohydrates and oxygen.
- Same as Natural process: Artificial photosynthesis (AP), is essentially conducting the same fundamental process in natural photosynthesis but with simpler nanostructures.
- Mechanism: It involved the immobilization of a photosensitizer.
- Which is a chemical called ruthenium bipyridyl complex ([Ru(bpy)2Cl2]).
- It also has a catalytic part which is another chemical called rhenium carbonyl complex ([Re(CO)5Cl]).
- Proximity: Both these molecular entities stay nearby in the nano-space of a porous metal-organic framework system resulting in excellent CO2uptake capability at room temperature.
- Photo-catalysis: This synthetic strategy empowers efficient solar light-driven photo-catalysis.
- Chemical Process:
- Reduction of CO2: The developed catalyst exhibited excellent visible-light-driven CO2reduction to CO with more than 99% selectivity.
- Water Oxidation: The catalyst also oxidizes water to produce oxygen (O2).